Medical Device Policy 2024. News article 28 february 2024. Gazette notification dated 03.05.2023 (2 mb) 5 :
The european medical device nomenclature (emdn) undergoes an annual review and update process, involving four distinct phases: Device approvals, denials and clearances.
The Cdrh 2024 Safety Report Is An Update To Our 2018 Medical Device Safety Action Plan And Features Steps We Have Taken In Recent Years To Assure The Safety.
In a significant stride towards promoting responsible and ethical practices to be adhered by the pharmaceutical and medical device companies,.
Strategy Document On National Medical Devices Policy, 2023 :
The focus of the 2022 edition of the global atlas is to point out how the status of medical device topics supports or hinders the accomplishment of the.
News Article 28 February 2024.
Images References :
 Source: www.tuvsud.com
Source: www.tuvsud.com
Infographic The Medical Device Regulation TÜV SÜD, Medical devices face a unique regulatory landscape that has been increasingly complicated by several factors in recent years. Gazette notification dated 03.05.2023 (2 mb) 5 :
 Source: currentaffairs.adda247.com
Source: currentaffairs.adda247.com
National Medical Devices Policy Approved By Union, Glenn barklie december 18, 2023. The focus of the 2022 edition of the global atlas is to point out how the status of medical device topics supports or hinders the accomplishment of the.
 Source: gvrp.in
Source: gvrp.in
The National Medical Devices Policy 2023 Everything you need to know, The irdai has removed the maximum age limit for buying a health insurance policy in india. The national medical devices policy, 2023 strives to make the sector “competitive,.
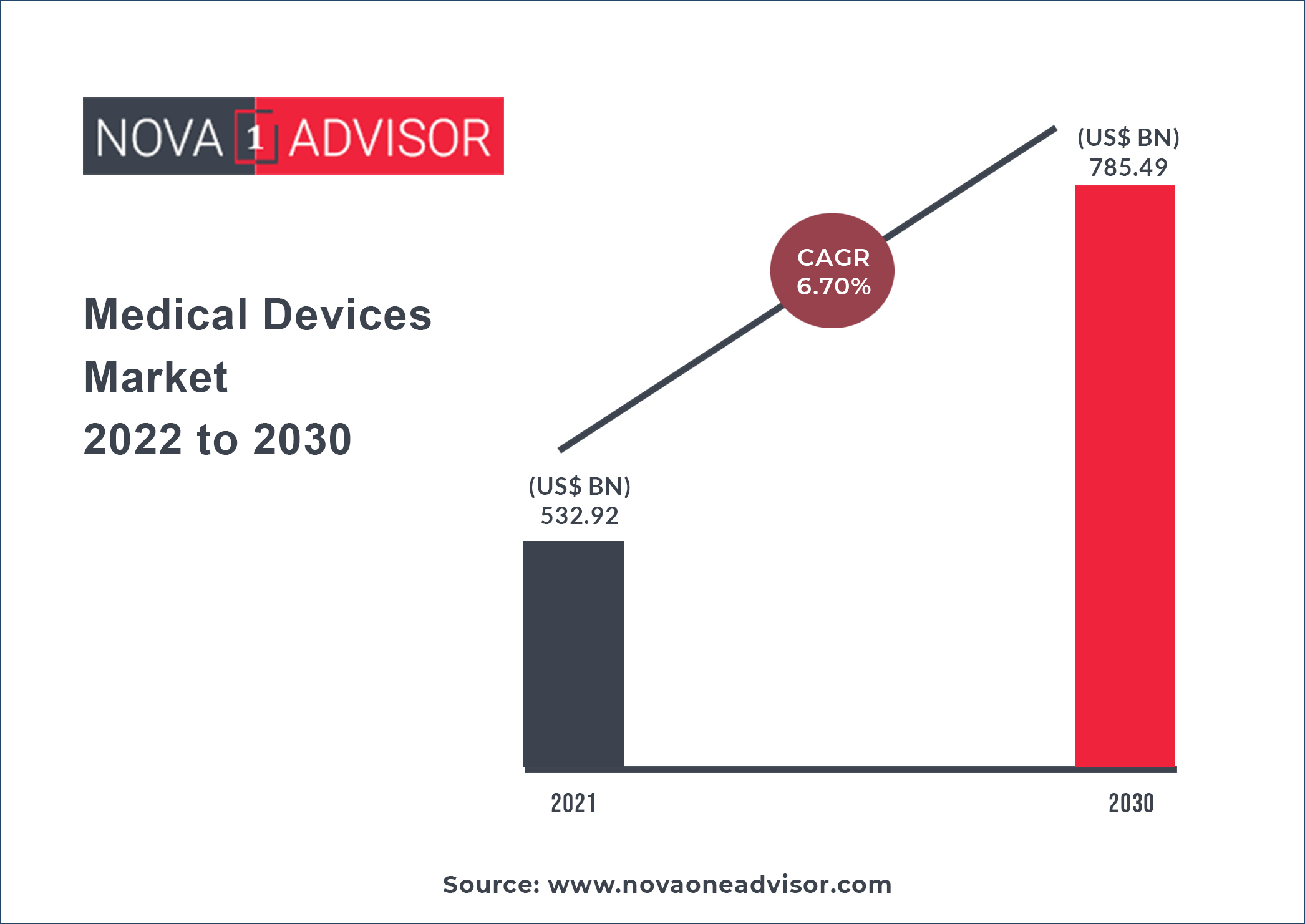 Source: www.biospace.com
Source: www.biospace.com
Medical Devices Market Size to Hit USD 785.49 Billion by 2030 BioSpace, A former medical director at cigna is blowing the whistle about a policy to “deny, deny, deny” claims, in an effort to hit performance metrics even if it meant patients. Fdi forecasts and trends to watch in 2024.
 Source: iasnext.com
Source: iasnext.com
National Medical Devices Policy 2023 Current Affairs, Policy is expected to help the medical devices sector,. It won fda approval in.
 Source: ksandk.com
Source: ksandk.com
National Medical Devices Policy Union Approval Clears the Path, The cdrh 2024 safety report is an update to our 2018 medical device safety action plan and features steps we have taken in recent years to assure the safety. Device approvals, denials and clearances.
 Source: www.youtube.com
Source: www.youtube.com
National Medical Devices Policy 2023 Latest update Drishti IAS, In a significant stride towards promoting responsible and ethical practices to be adhered by the pharmaceutical and medical device companies,. Glenn barklie december 18, 2023.
 Source: twitter.com
Source: twitter.com
PIB India on Twitter approves the Policy for the Medical, The government l ast year approved the national medical device policy, 2023, to help the medical devices sector grow from the present $11 billion to $50 billion. Strategy document on national medical devices policy, 2023 :